G-CSFR is a cell surface receptor that can activate cell proliferation and differentiation after binding to granulocyte colony stimulating factor(G-CSF). However, in natural killer (NK) cells, the biological role of G-CSFR has not been fully elucidated. Here we show that granulocyte colony-stimulating factor receptor, that is encoded by the CSF3R gene, is a novel and potent checkpoint in both human and mouse NK cells fighting against tumors.
Previous studies have shown that NK cells under resting state with low expression of G-CSFR, but highly expressed in peripheral blood and bone marrow NK cells after G-CSF mobilization. We therefore used G-CSFR blocking agent in vitro to evaluate the effect of G-CSFR on human NK cells proliferation and cytotoxicity capacity. The results showed that G-CSFR agonist significantly down-regulated the proliferation and cytotoxicity of NK cells in vitro and this is independent of G-CSF stimulation. Since NK92 and YT cell lines highly express G-CSFR, we then knocked down G-CSFR in NK92 and YT cells. The results showed that the low expression of G-CSFR enhanced the expression of CD107a in vitro, indicating G-CSFR may be an inhibitory signaling in NK cells.
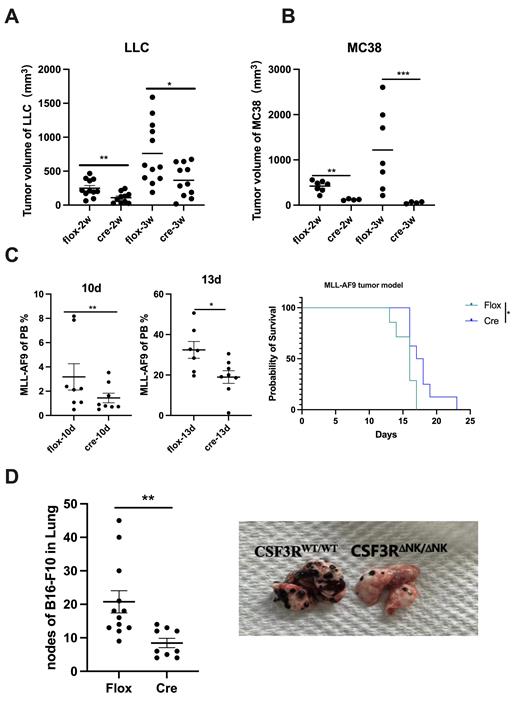
To the further evaluate the specific role of G-CSFR in NK cells and we therefore constructed NK-specific CSF3R deficiency mice (CSF3R △NK/NK; CSF3Rfl/flNCR1cre, thecre group) and the control group ( CSF3Rfl/fl ( CSF3RWT/WT mice, the flox group). We then evaluated the phenotype, differentiation and functional state of NK cells between the two group. NK-specific CSF3R deficiency increased mature NK subsets in mice, and these CSF3R-deficient NK cells exhibited enhanced activation, cytotoxicity and proliferation, which is in accordance with the role in human NK cells. After constructing four tumor-bearing models, namely MC38 (colon tumor), LLC (lung tumor), MLL-AF8(acute myeloid leukemia), B16-F10(melanoma), we found that CSF3R specific depletion in NK cells reduced the tumor burden, prolonged the survival and reduced tumor metastasis in CSF3R△NK/NK mice (Figure 1A-D), indicating CSF3R depletion in NK cells promotes increased ability against tumors.
To further evaluate the molecular mechanism of G-CSFR regulation of NK cell function, single cell RNA sequencing on bone marrow cells from both CSF3R△NK/NK mice and CSF3RWT/WT mice and NK cells were extracted for further subpopulation analysis. UMAP analysis revealed the clustering of bone marrow NK cells from both genotypes into ten subsets and on the basis of the key gene expression of NK cell effector molecules, transcriptional factors, maturation markers, and proliferation marker, we identified NK cell maturation program as mBM5+mBM8, mBM2+mBM3+ mBM6 and mBM0, by which immature NK cells should go through to gain optimal effector function, according to this model, CSF3R△NK/NK mice contained more NK cells belonging to the terminally mature mBM0 than CSF3RWT/WT mice. “Cell Killing” and “leukocyte mediated cytotoxicity” pathway was enriched in CSF3R△NK/NK mice, indicating enhanced activation state of NK cells. In conclusion, our results uncover a potent checkpoint in NK cells protecting against tumors, suggesting a promising approach of targeting G-CSFR for NK cell-based immunotherapies.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal